The U.S. Food and Drug Administration announced today the approval of Jynneos Smallpox and Monkeypox Vaccine, Live, Non-Replicating, for the prevention of smallpox and monkeypox disease in adults 18 years of age and older determined to be at high risk for smallpox or monkeypox infection. This is the only currently FDA-approved vaccine for the prevention of monkeypox disease.
Read full statement by FDA: https://www.fda.gov/news-events/press-announcements/fda-approves-first-live-non-replicating-vaccine-prevent-smallpox-and-monkeypox
“Following the global Smallpox Eradication Program, the World Health Organization certified the eradication of naturally occurring smallpox disease in 1980. Routine vaccination of the American public was stopped in 1972 after the disease was eradicated in the U.S. and, as a result, a large proportion of the U.S., as well as the global population has no immunity,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “Therefore, although naturally occurring smallpox disease is no longer a global threat, the intentional release of this highly contagious virus could have a devastating effect. Today’s approval reflects the U.S. government’s commitment to preparedness through support for the development of safe and effective vaccines, therapeutics, and other medical countermeasures.”
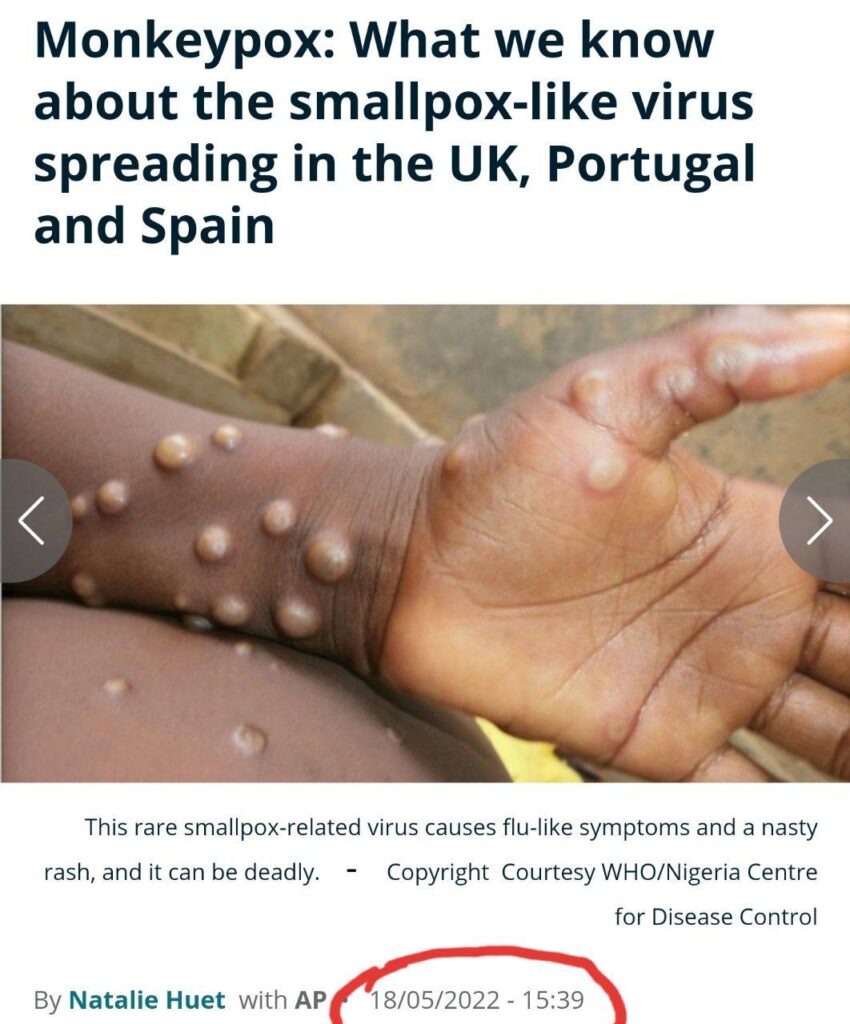
…and in other news, there are these coincidental stories!
Subscribe
Click here for a secure way to sign up, you will be supporting independent news. Click the button below.
Your Opinions
Disagree with this article? why not write in and you can have your say? email us